Int. J. Innov. Entrep. 2022, 1(1), 2; doi:10.56502/IJIE1010002
Essay
Advanced Oxidation Processes for Water Purification Applications
Lord Byng Secondary School, 3939 West 16th Avenue, Vancouver, BC V6R 3C9, Canada; 2157525@learn.vsb.bc.ca
How to Cite: Tao, Y. Advanced Oxidation Processes for Water Purification Applications. Int. J. Innov. Entrep., 2022, 1(1): 2; doi:10.56502/IJIE1010002.
Received: 20 November 2022 / Accepted: 1 December 2022 / Published: 8 December 2022
Abstract
:The contamination of water resources has become a significant challenge that threatens the health and well-being of people’s daily life; it calls for new technologies to be applied for water purification. Heterogeneous Fenton or Fenton-like techniques have a lot of potential as a new approach for treating wastewater because of the advantages in efficiency, safety, and economics. Various advanced oxidation processes (AOPs) are comprehensively reviewed in this work, and the •OH generation methods including chemical, electro-chemical, and photochemical approaches are summarized. Some key parameters that influence the reaction efficiency such as the temperature, catalysts, H2O2 dosage, and pH are investigated. The reaction mechanism and process optimization of photocatalysis are specifically highlighted.
Keywords:
catalysis; Fenton reaction; electron-Fenton; photocatalysis; hydroxyl radicalsThere is an increasing demand for fresh water due to the growing world population. However, the rapid development of industrialization and the economy has brought about significant water pollution issues which have intensified the conflicts associated with water supply problems worldwide (Peydayesh and Mezzenga 2021). The massive discharge of industrial wastewater has also posed a serious threat to humans and the whole eco-system (Schwarzenbach et al. 2010). Organic contaminants such as dyes, antibiotics, and pesticides are often characterized as having high toxicity, high chemical stability, and poor biodegradability, the majority of which are persistent organic pollutants (POPs) and easily accumulate in the environment (Schwarzenbach et al. 2010). Thus, the development of techniques that are capable of efficient removal of these compounds is imperative.
Conventional disposal methods could hardly provide a balancing approach for both cost and performance (Chen et al. 2020). That is, traditional biological processes are not effective for biorefractory contaminants, yet the application of tertiary treatments such as adsorption, ion exchange, and membrane separation are often limited by their high cost and secondary pollution (Chen et al. 2020). In recent years, the development of advanced oxidation processes (AOPs) has provided a promising alternative for organic wastewater treatment, especially for POPs, due to their high performance, simplicity, and environmental compatibility (Guo et al. 2020).
AOPs mostly refer to the techniques that involve the in situ generation of hydroxyl radicals (•OH) through catalysis, which could unselectively oxidize the organics (Pandis et al. 2022). •OH, a strong oxidant that acts as the main reactive oxygen species (ROS), could behave as a highly reactive electrophile and decompose the organics into small and stable molecules, which could even be mineralized into H2O and CO2, by breaking aromatic rings and hydrocarbons via hydrogen abstraction and some additional processes (Pandis et al. 2022).
In general, AOPs could be classified according to the •OH generation methods including chemical, electro-chemical, and photochemical approaches, etc. Fenton or Fenton-like reactions are kinds of commonly applied chemical processes (Pandis et al. 2022). The mixed solution of Fe2+ and H2O2 was named a Fenton reagent in memory of Fenton’s contribution to this process (Babuponnusami and Muthukumar 2014). Haber, Weiss, and Walling et al. suggest that •OH is the dominant ROS that accounts for the organic decomposition (Babuponnusami and Muthukumar 2014). Their research indicated that the Fenton reaction depends on the redox process of Fe2+-Fe3+ to catalyze H2O2 to produce •OH and other reactive oxygen groups such as superoxide radicals (O2•−) and singlet oxygen (1O2), among which •OH radicals have the highest oxidation potential of 2.8 V and contribute the most to the degradation with a reaction constant rate of 106–109 L/(mol•s) (Babuponnusami and Muthukumar 2014). This is the basis of the current classical radical Fenton reaction theory, which can be summarized in Equations (1)–(8) (RH stands for the organic contaminants). The reduction step of Fe3+ to Fe2+ in Equation (4) is the rate-limiting step for the whole reaction (Babuponnusami and Muthukumar 2014).
Fe2+ + H2O2 → Fe3+ + •OH + OH−
Fe3+ + H2O2 → Fe2+ + HO2•+ H+
Fe2+ + •OH → Fe3+ + OH−
Fe3+ + HO2• → Fe2+ + O2 + H+
H2O2 + •OH → H2O + HO2•
•OH + RH → R• + H2O
R• + Fe3+ → R+ + Fe2+
R+ + O2 → ROO+ → ••••• → CO2 + H2O
Yet there is also another kind of explanation for the mechanism of Fenton reactions. Kremer believes that the high valent Fe, i.e., Fe (IV, V, VI), formed during Fenton reaction is the key driver for the degradation according to Equation (9) (Kremer 2000). The peroxy complex intermediate of high-valent iron and organic compounds is significant for the process (Kremer 2000).
Fe2+ + H2O2 + H+ → Fe(IV, V, VI)/Fe
In addition to Fe2+, other metal ions such as Fe3+, Cu2+, Co2+, Mn2+, Ag+ and some iron-based minerals have been found to be capable of accelerating or replacing Fe2+ in H2O2 catalysis; these reactions are named Fenton-like reactions (Guo et al. 2020, 2022). However, traditional homogeneous Fenton or Fenton-like reactions were severely limited, especially due to the constraints of impractical low pH values (pH = 3–5), poor recyclability of catalysts, and sludge accumulation after neutralization (Guo et al. 2020, 2022). As a result, the heterogeneous process with high degradation efficiency and easy-recycling catalysts of good stability has drawn growing attention as a promising alternative both in academic and industrial fields (Guo et al. 2020). Mostly, the reactions could be carried out at ambient temperature and pressure, and the degradation performance could be greatly influenced by parameters such as the catalyst dosage, reaction temperature, pH of surroundings, and H2O2 dosage (Guo et al. 2020, 2022). Specifically, low-cost transition-metal-based catalysts with high activity have drawn a broad range of attention. For example, Guo et al. proved Co-Cu LDH to be an efficient catalyst for the disposal of anthraquinones-containing H2O2 production effluent with COD (chemical oxygen demand) and TOC (total organic carbon) removal of 89.9% and 71.3%, respectively (Guo et al. 2020).
As to the electro-chemical treatment, electron-Fenton (EF) has also attracted great interest with the application for treating hazardous waste. In the EF process, H2O2 could be in situ generated by the cathodic reduction of O2 according to Equation (10), which could then react with externally added Fenton or Fenton-like catalysts to produce •OH via a homogeneous or heterogeneous process (Babuponnusami and Muthukumar 2014; Wang et al. 2022). Recently, Fe-modified carbon/graphite felt and transition-metal-doped carbon aerogel were developed into functionalized cathodic materials as working electrodes (Wang et al. 2022). In addition to the advantages of heterogeneous catalysts that are mentioned above, the cathodic materials could be recovered and reused easily with the elimination of the potential danger for the long-distance transportation of H2O2. The overall disposal cost could thus be reduced. The performance of the EF catalytic process is mostly determined by the parameters of the electrode nature, pH of surroundings, temperature, catalyst dosage, electrolytes, current density, and dissolved oxygen level (Babuponnusami and Muthukumar 2014; Wang et al. 2022). For the EF treatment of leather tanning industry wastewater, Brillas et al. achieved a COD removal of 60% at neutral pH with an energy demand of 3.8 kWh/g COD removal (Brillas and Casado 2022).
2H+ + O2 + 2e → H2O2
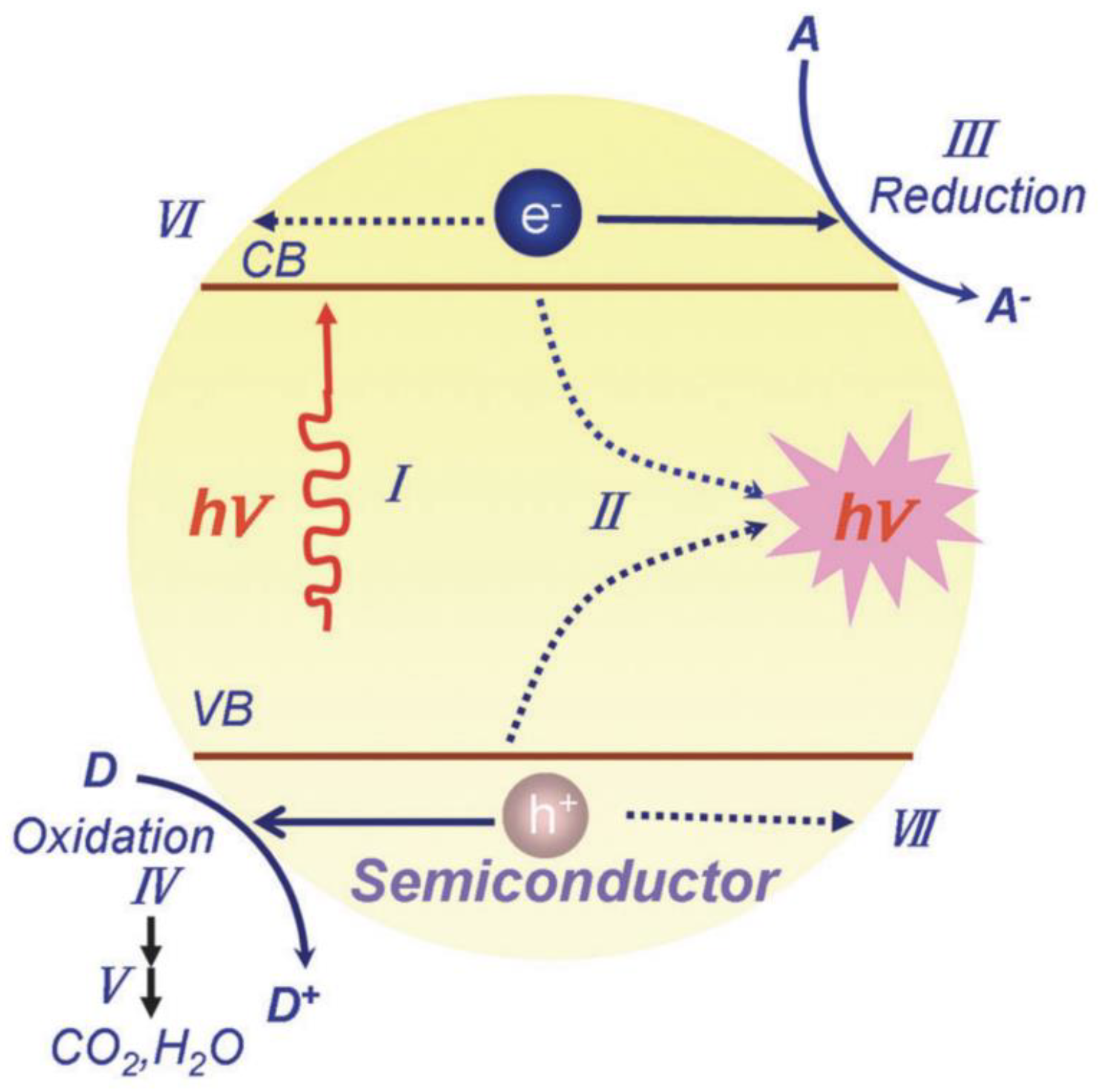
In addition to the above techniques, photocatalysis is probably one of the most popular approaches for water purification. The mechanism of photocatalysis is as shown in Figure 1 (Wang et al. 2014). In most cases, photocatalysts are semiconductor materials which could produce electrons and holes on exposure to ultraviolet or visible radiation (Brillas and Casado 2022). An electron in a filled valence band absorbs photonic energy greater than the energy of the band gap from the radiation, resulting in the formation of an electron–hole (h+) in the original valence band (VB) and the excitation of the electron (e−) to the vacant conduction band (CB), as depicted in Process I in Figure 1 (Wang et al. 2014). These charge carriers in the CB (e−) and VB (h+) could initiate reduction (Process III in Figure 1) and oxidation (Process IV in Figure 1) reactions with substrates adsorbed on the catalysts’ surface, respectively, or they may recombine with each other without any chemical reaction proceeding (Process II in Figure 1) (Wang et al. 2014). Concretely, h+ could oxidize H2O or OH− into •OH while e− could reduce O2 into O2•−, which could both contribute to organic degradation (Perović et al. 2020). Metal oxides, in general, have the most diverse applications in the AOPs field, among which TiO2 (Perović et al. 2020), ZnO (Youssef et al. 2018), and other binary metal oxides (Davarikia et al. 2022) are most widely used.
Overall, AOPs, especially the heterogeneous ones, are highly efficient methods for wastewater treatment; these techniques have great potential for industrial applications. The development of efficient and cheap AOP catalysts can greatly reduce the cost of the application of corresponding technology and effectively promote the catalytic performance of organic degradation. Therefore, the development of these catalysts is highly important for both academic research and industrial applications for water purification.
Conflicts of Interest
The author declares no competing financial interests.
References
- Babuponnusami, Arjunan; Muthukumar, Karuppan. A review on Fenton and improvements to the Fenton process for wastewater treatment. Journal of Environmental Chemical Engineering 2014, 2, 557. [Google Scholar] [CrossRef]
- Brillas, Enric; Casado, Juan. Aniline degradation by electro-Fenton and peroxi-coagulation processes using a flow reactor for waste water treatment. Chemosphere 2022, 47, 241. [Google Scholar] [CrossRef] [PubMed]
- Chen, Dongjie; Cheng, Yanling; Zhou, Nan; Chen, Paul; Wang, Yunpu; Li, Kun; Huo, Shuhao; Cheng, Pengfei; Peng, Peng; Zhang, Renchuang; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. Journal of Cleaner Production 2020, 268, 121725. [Google Scholar] [CrossRef]
- Davarikia, Yasaman; Aroujalian, Abdolreza; Salimi, Parisa. Immobilization of TiO2 nanoparticles on PES substrate via dopamine and poly (vinyl alcohol) for long-term oil/water purification. Process Safety and Environmental Protection 2022, 166, 656. [Google Scholar] [CrossRef]
- Guo, Xiaoxi; Hu, Tingting; Meng, Bo; Sun, Yanf; Han, Yi-Fan. Catalytic degradation of anthraquinones-containing H2O2 production effluent over layered Co-Cu hydroxides: Defects facilitating hydroxyl radicals generation. Applied Catalysis B: Environmental 2020, 260, 118157. [Google Scholar] [CrossRef]
- Guo, Xiaoxi; Hu, Bo; Wang, Ke; Wang, Huanhuan; Li, Bolan; Guo, Mai; Tian, Yun; Zhang, Ruixue; Shi, Shishuai; Han, Yifan. Cu embedded Co oxides and its Fenton-like activity for metronidazole degradation over a wide pH range: Active sites of Cu doped Co3O4 with 1 1 2 exposed facet. Chemical Engineering Journal 2022, 435, 132910. [Google Scholar] [CrossRef]
- Kremer, Mordechai L. Is OH the active Fenton intermediate in the oxidation of ethanol? Journal of Inorganic Biochemistry 2000, 78, 255. [Google Scholar] [CrossRef] [PubMed]
- Pandis, Pavlos K.; Kalogirou, Charalampia; Kanellou, Eirini; Vaitsis, Christos; Savvidou, Maria G.; Sourkouni, Georgia; Zorpas, Antonis A.; Argirusis, Christos. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Perović, Klara; Rosa, Francis M. dela; Kovačić, Marin; Kušić, Hrvoje; Štangar, Urška Lavrenčič; Fresno, Fernando; Dionysiou, Dionysios D.; Bozic, Ana Loncaric. Recent achievements in development of TiO2-based composite photocatalytic materials for solar driven water purification and water splitting. Materials 2020, 13, 1338. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, Mohammad; Mezzenga, Raffaele. Protein nanofibrils for next generation sustainable water purification. Nature Communications 2021, 12, 3248. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, René P.; Egli, Thomas; Hofstetter, Thomas B.; Gunten, Urs von; Wehrli, Bernhard. Global water pollution and human health. Annual Review of Environment and Resources 2010, 35, 109. [Google Scholar] [CrossRef]
- Wang, Huanli; Zhang, Lisha; Chen, Zhigang; Hu, Junqing; Li, Shijie; Wang, Zhaohui; Liu, Jianshe; Wang, Xinchen. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chemical Society Reviews 2014, 43, 5234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Zining; Liu, Mingyue; Xiao, Fan; Postole, Georgeta; Zhao, Hongying; Zhao, Guohua. Recent advances and trends of heterogeneous electro-Fenton process for wastewater treatment-review. Chinese Chemical Letters 2022, 33, 653. [Google Scholar] [CrossRef]
- Youssef, Zahraa; Colombeau, Ludovic; Yesmurzayeva, Nurlykyz N.; Baros, F.; Vanderesse, Regis; Hamieh, Tayssir; Toufaily, Joumana; Frochot, Celine; Roques-Carmes, Thibault; Acherar, Samir. Dye-sensitized nanoparticles for heterogeneous photocatalysis: Cases studies with TiO2, ZnO, fullerene and graphene for water purification. Dyes and Pigments 2018, 159, 49. [Google Scholar] [CrossRef]
Figure 1.
Mechanism of photocatalysis. The figure has been reproduced with the permission of Wang et al. 2014 (Copyright @ The Royal Society of Chemistry 2014).
Figure 1.
Mechanism of photocatalysis. The figure has been reproduced with the permission of Wang et al. 2014 (Copyright @ The Royal Society of Chemistry 2014).
© 2022 Copyright by Authors. Licensed as an open access article using a CC BY 4.0 license.
